| |
Reinforcing Revenues |
| At Dr. Reddy's, it is our constant endeavour to hone our competitive edge and promote access to affordable, innovative medicines across the globe. We think beyond set norms and outside the box to unearth opportunities that meld affordability for people everywhere with revenues for the organisation. |
| |
| Prolific Pipeline |
| Authorised generics, such as for the cholesterol drugs Zocar and Proscar or the recently launched sumatriptan, have made significantly large contributions to our revenues. The launch of sumatriptan, the authorized generic version of Imitrex®, ahead of others in the US, contributed INR 7,188 million in 2008-09 and INR 2,543 million in 2009-10. |
|
| We have invested in our generics future. Dr. Reddy's has 38 Para-IV filings in the US, with 12 in the category of ‘first to file'. This potential is backed by a proven prowess in complex chemistry. If even one or two of these filings fructify every year, a healthy revenue pipeline will be assured. |
|
| As per the US rules, when a generic company successfully challenges an innovator patent and has first-to-file challenge status, it gets 180 days' exclusivity to sell the product in that country. |
|
| In 2009-10, we filed 13 drug applications in North America, of which 12 Abbreviated New Drug Applications (ANDAs) were in the US and one in Canada. Among these were six Para-IV filings. The US generic pipeline now comprises 73 ANDAs pending with the USFDA, including 11 tentative approvals. As of 31 March 2010, we have filed 179 cumulative drug applications in both the US and Canada. |
|
| In the Active Ingredients business, we filed 36 Drug Master Files (DMFs) in 2009-10. |
|
 |
|
| As on 31 March 2010, Dr. Reddy's had cumulative filings of 378 DMFs, with 156 in the US. |
| |
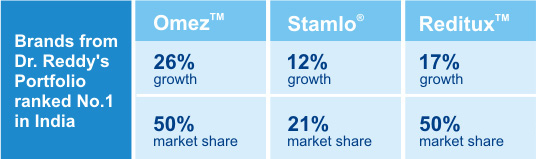
| Brand Power |
| Dr. Reddy's top-10 brands accounted for revenues of INR 3,845 million (38% of India formulations revenue). The ability to build brand leaders demonstrates our capability to create a preference for our products in the marketplace. The resultant brand equity ensures steady and predictable revenues. |
|
|
| |
 |
| |
| Local Leverage |
| Dr. Reddy's constantly strives to leverage local resources by generating employment for regional populace as well as sourcing goods and services from local geographies. We have successfully nurtured relationships with indigenous business partners for key raw material supplies such as isopropyl and pantaprozole. This not only gives us greater control over the supply of raw material and better cost efficiency, but also gives our local suppliers and vendors the opportunity to integrate with the global markets. |
| |
| Unlocking Profits |
| Considering the demand cycle and the large product range that we offer, it becomes imperative to maintain inventory in order to serve the markets better. At times, this leads to situations where some stock becomes stagnant and runs the risk of expiry. This year, with an aim to optimize inventory levels, we set up a team to identify non-moving inventory, and to transfer or sell it at extremely affordable rates to markets where there was a demand for it. These markets were strategically chosen keeping in mind the existing product demand so that there was no cannibalism of our own products. The exercise not only benefited hundreds of patients by providing them access to even more affordable medicine, but also netted the organisation approximately INR 60-100 million. |
| |
| Raising the Bar |
| We are a dynamic organisation – one that learns from its mistakes and takes preemptive measures to avoid their recurrence in the future. We constantly undertake root cause analysis of processes and events to enhance quality, reliability and speed. |
| |
| Application Accelerator |
| Under this initiative we highlighted and fine-tuned a key process - the filling of DMF (Drug Master File) and other USFDA applications. A large knowledge bank was constituted to ensure that there is zero or only minor observations in our filings. The core objective was to speed up the process and increase time and resource efficiency. |
|
| We understand that organisational growth and the ever changing macro environment lead to new demands on existing processes. These changes can create gaps or growth bottlenecks. To better address these problems we constantly track the future readiness of our processes across functions and undertake transformational initiatives to fundamentally improve their performance. |
|
| Function |
Challenges |
Initiatives |
|
|
|
| Marketing |
Quality of delivery especially
during demand spikes |
Ensuring adequate buffer
stock; Ramping up the scope
& horizon of the planning process |
| Manufacturing |
Compensating for sudden
surges in demand; managing
the manufacturing through third
party vendors, if necessary |
Comprehensive training, handholding and mentoring
of new suppliers, ensuring adequate capacity in their manufacturing facilities |
| Project Management |
Procurement of materials
(especially during times of
booming demand) |
Timely capital expenditure in order to preempt such situations |
External Authorities
and Governing Bodies |
Liaisoning with Government
Bodies (especially Pollution
Control Boards) |
Becoming a part of body of organisations. For example, Bulk Drug Manufacturers Council etc. |
| Stores |
Storage space; coordinating with
new employees or third party
workers |
Ongoing capacity expansion; training for newly employed
and third party workers |
|